It was a gloomy, rainy October day in 2007 when Kathryn began to see in colour again. This day marked the moment she started to recover — from a 19-year battle with a profoundly disabling treatment-resistant depression.
Kathryn had gone shopping early that morning. When she left the mall and walked outside, all she saw were the colours of the leaves on the trees.
“Seeing these blazing reds, intense yellows and oranges in a way that I hadn’t seen in years,” said Kathryn. “I started to sob right in that parking lot.”
Severe depression is insidious. Kathryn describes it not as an intense sadness, but as a numbness — the inability to feel anything at all. According to the World Health Organization, depression is the leading cause of disability worldwide. And research on treatment-resistant depression shows that one third of people don’t respond to trials of multiple medications and treatment, such as psychotherapy and electroconvulsive therapy.
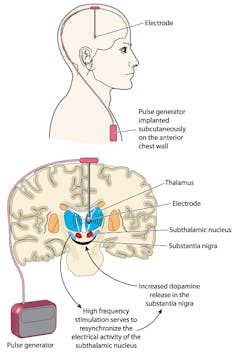
The day Kathryn’s senses came “back online” was two years, two months and five days after undergoing an experimental brain surgery — known as deep brain stimulation (DBS).
But before the Food and Drug Administration can approve DBS, clinical trials must show that it is safe and can effectively fight chronic cases of treatment-resistant depression.
Turning down the sadness
Physicians started experimenting with DBS for chronic, treatment-resistant depression in 2003. In this procedure, a surgeon drills a small hole into the skull and directs an electrode down into a region called Brodmann area 25 (BA25) — what Dr. Andres Lozano, a neurosurgeon at Toronto Western Hospital and leader in the field of DBS, has referred to as the “sadness centre of the brain.”
The other end of the electrode is tunnelled down under the skin to a battery, or pacemaker-like device, in the chest. Doctors then use a remote control to turn up or down — like the volume on a speaker — the amount of electricity delivered to that area of the brain.
While DBS refers to “stimulation,” it is actually an inhibitory effect that is at work. The current from the electrodes are turning down sadness, pumping the brakes. In people with treatment-resistant depression this area of the brain is very active and medication or psychotherapy can’t quieten it down.
Like most surgeries, DBS still carries risks, but it is now an approved treatment for Parkinson’s disease and essential tremor.
Failed trial, successful treatment?
DBS is not a cure. If stimulation stops, the depression will return. It’s also not a quick-fix. DBS puts the brakes on sadness and, over time, sets other areas of the brain in drive, but the real work happens afterwards.
In October, the journal Lancet Psychiatry published new data on the largest, prospective study of DBS for any psychiatric disorder.
The summary of the study confirmed the safety of the procedure but did not show significant success in reducing depression at the six- and 12-month period. A closer look at the research’s long-term data tells a different story.
St. Jude Medical, the company that sponsored the trial estimated that the study had a low likelihood (17 per cent chance) of success if it continued, and so it stopped recruiting new participants. (St. Jude Medical was acquired by Abbott earlier this year.) “It wasn’t going as they expected. It’s very expensive. And they made a business decision to stop the trial,” said neurologist Dr. Helen Mayberg of Emory University in Atlanta.
Ninety participants initially took part in the study and were randomly assigned to two groups — 60 of them receiving active stimulation, the other 30 receiving no stimulation. Patients did not know which group they belonged to.
Initially the BROADEN trial found no significant difference in efficacy between the group receiving stimulation and the group in which the device was implanted but not turned on. But, as Dr. Mayberg said, “a failed trial is not a failed treatment.”
There was a second phase of the research, where 77 of the initial 90 patients entered into a four-year follow-up. In this part of the study all patients received treatment. After two years, nearly half of all patients had responded to the therapy — 48 per cent (25 patients) achieved an antidepressant response and 25 per cent (13 patients) achieved remission.
This seems to suggest that “it may take longer to get better the more chronically ill you are,” said Dr. Mayberg. In her own research, Dr. Mayberg said that unless the DBS system breaks, or has to be removed because of infection, “we actually don’t have people ever going back to their pre-surgical state.”
That being said, more compelling evidence is needed to make any real case for DBS. That will require additional studies — which means more patients.
‘This is not a pill’
In the BROADEN trial, 28 patients experienced 40 serious adverse events during the first year of the study; eight of these (in seven patients) were related to the device or surgery. Complications included infection, skin erosion and one postoperative seizure. Other serious adverse events were related to the treatment-resistant depression itself. There were two deaths by suicide.
Patients in the BROADEN trial had the most severe cases of treatment-resistant depression, being already 12 years into their depressive episode when they joined. This may explain the slowed response shown in the trial. The placement of the electrodes in DBS is also important to the surgery’s success. Thirteen surgical centres participated in the trial, so there were differences in how the surgery was performed from centre to centre.
“This is not a pill, it’s a surgical procedure,” said Dr. Lozano, “so there’s much more variability in the surgical procedure than there is in taking a simple medicine.” Following this study, more targeted approaches to the placement of DBS electrodes have since been developed.
The real story is the one that comes after surgery. When the hype of a new treatment — or failed treatment — subsides, life continues on for the patients.
Of those patients enrolled in the BROADEN trial, 44 are still being treated today and getting their DBS batteries changed, on average, every three to five years.
When I reached out to Abbott (the sponsors of the trial) they informed me that they continue to provide device support to the trial patients that kept their DBS implants in, and have agreed to supply them with rechargeable batteries. If a patient wants the system removed, Abbott has said it will pay.
Rebuilding a life
Kathryn, one of Dr. Mayberg’s first patients (and not part of the BROADEN trial), is one of those for whom DBS has worked. Her road to recovery hasn’t been easy. Prior to her DBS surgery, she had tried over 40 different medications, electroconvulsive therapy and psychotherapy. Kathryn had very real plans of suicide throughout the years before her surgery.
“Recovery is actually a very long process. It’s not like you just wake up one morning and your depression is gone and your life is wonderful,” said Kathryn. It’s been 10 years now since that momentous October morning when the grey lifted. Today, Kathryn doesn’t take medication and has no residual symptoms of depression. She so far has had no adverse effects to DBS.
Her case is unusual. Most patients need some form of medication. Kathryn wants others considering this road to have realistic expectations.

Just as after any surgery, there is rehabilitation after DBS. When someone has their hip replaced they must learn to walk again, said Dr. Peter Giacobbe, a Toronto psychiatrist at the University Health Network who works with DBS patients and worked closely with Kathryn.
It’s the same with surgery for depression, “these people need to learn how to rebuild their lives and undo past ways of connecting with others,” he said. “The people who successfully do that, you can see that they’re able to go back to work. Their remission rates creep up over one to three years.”
As for Kathryn, today she lives a very full life. She works, volunteers, sits on a number of committees and is involved with patient-engagement work related to mental health. It’s quite a change for someone who once struggled to leave her bed.
‘Til device or death do you part’
“DBS is a long-term prospect,” said Dr. Giacobbe. “We work with these people for 15 years [or more]. You get married to them in some ways … it’s ‘til device or death do you part.”
For now, DBS remains an experimental surgery. While it is reserved for the most severe cases of depression, Kathryn refers to the power that comes from just knowing that something like DBS is out there, and that other potential new solutions are emerging, such as genetic testing and new medications inspired by the drug ketamine.
DBS, because it’s a surgery, also has the opportunity to further destigmatize mental illness, said Dr. Giacobbe, “by showing people that a physical intervention can help an emotional disorder.”
In the meantime, while we wait, we have hope. And for those struggling, hope remains a most powerful medicine.
Author:Kylie Gionet Munk Journalism Fellow/Registered Nurse, University of Toronto
Credit link:https://theconversation.com/could-an-experimental-brain-surgery-make-you-happier-88060<img src="https://counter.theconversation.com/content/88060/count.gif?distributor=republish-lightbox-advanced" alt="The Conversation" width="1" height="1" />

