Manal Mohammed, University of Westminster and Andrew Millard, University of Leicester
The world is in the midst of a global “superbug” crisis. Antibiotic resistance has been found in numerous common bacterial infections, including tuberculosis, gonorrhoea and salmonellosis, making them difficult – if not impossible – to treat. We’re on the cusp of a “post-antibiotic era”, where there are fewer treatment options for such antibiotic-resistant strains. Given estimates that antibiotic resistance will cause 10 million deaths a year by 2050, finding new methods for treating harmful infections is essential.
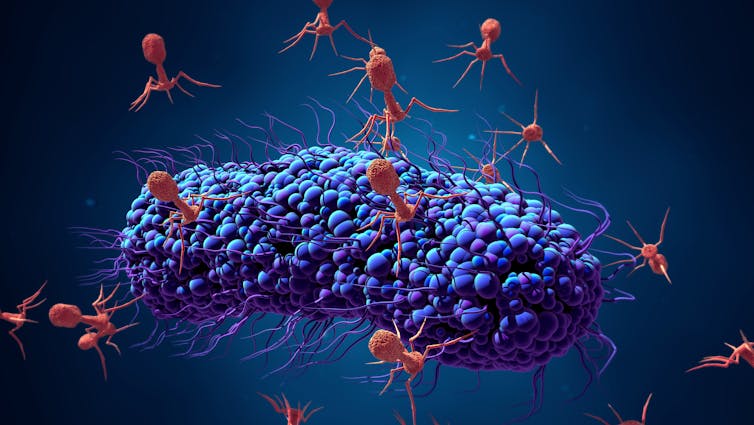
Strange as it might sound, viruses might be one possible alternative to antibiotics for treating bacterial infections. Bacteriophages (also known as phages) are viruses that infect bacteria.
They’re estimated to be the most abundant organisms on Earth, with probably more than 1031 bacteriophages on the planet. They can survive in many environments, including deep sea trenches and the human gut. While phages are efficient killers of bacteria, they don’t infect human cells and are harmless to humans.
Although phage therapy was used in the 1930s, it has since become a forgotten cure in the west. Although the treatment became commonplace in the former Soviet Union, it wasn’t adopted by western countries largely because of the discovery of antibiotics, which became widespread after World War II.
Bacteriophages are effective against bacteria because they’re able to attach themselves to the cell if they recognise specific molecules called receptors. This is the first step in the “infection” process. After attaching to the bacterial cell, the phage then injects its DNA inside the bacteria.
This causes one of two things to happen. After being injected with the phage’s DNA, the virus will take over the bacterial cell’s replication mechanism and start producing more phages. This process is known as a “lytic infection”. This disintegrates the cell, allowing the newly produced viruses to leave the host cell to infect other bacterial cells.
But sometimes, the phage DNA gets incorporated into the bacterial host’s chromosome instead, becoming a “prophage”. It usually remains dormant but environmental factors, such as UV radiation or the presence of certain chemicals such as those found in sunscreen, can cause the phage to “wake up”, start a lytic infection, take over the host cell and destroy it.
Lytic bacteriophages are preferred for treatment because they don’t integrate into the bacterial host’s chromosome. But it’s not always possible to develop lytic bacteriophages that can be used against all types of bacteria. As each type of phage is only able to infect specific types of bacteria, they can’t infect a bacterial cell unless the bacteriophage can find specific receptors on the bacterial cell surface.
However, engineering techniques can remove the bacteriophage’s ability to integrate into the host’s genome, making them useful for treatment. Engineered phages have even successfully treated a drug-resistant Mycobacterium abscessus infection in a 15-year-old girl.
Targeted treatment
The reason bacteriophages are so effective against bacteria is because they’re only able to infect specific species. Antibiotics instead target a wide range of bacteria, including “friendly” bacteria not causing the infection.
But this also means that a single phage won’t kill all strains of a disease-causing bacteria. And because bacteria are constantly evolving, they can develop mechanisms that prevent phage infection. For example, if the bacterial cell has evolved and changed its surface receptors, the bacteriophage won’t be able to attach itself and kill the bacteria.
As part of this evolutionary process, bacteria can rapidly become resistant to a single bacteriophage. But because there are many types of bacteriophages, we can use a “phage cocktail” containing a combination of different bacteriophages to target a broader range of bacterial strains within a species. This decreases the chances a bacteria becomes resistant to all phages used in treatment. Bacteriophages can also be engineered to infect more strains of bacteria.
However, the presence of what are known as CRISPR systems might complicate the possibility of using bacteriophages in treatment. CRISPR is a bacteria’s natural defence system that allows it to become immune to genetic material, such as phages, through infection, vaccination or the transfer of antibodies. Bacteria may be resistant to bacteriophages if they have previously encountered similar types and developed immunity.
But bacteriophages have also developed anti-CRISPR proteins that can neutralise the host bacteria’s CRISPR systems. This means a phage can still be effective, despite the presence of the bacterial CRISPR system. Not all bacteriophages have genes that neutralise anti-CRISPR proteins. But with the ability to engineer phage genomes, these could be incorporated into phages that are to be used for treatment in the future.
Read more: Soviet-era treatment could be the new weapon in the war against antibiotic resistance
Although phage therapy isn’t routinely used in western medicine, phage cocktails are available treatments in Russia and Georgia. Phage therapy is also a common part of medical care in Georgia, especially in paediatric, surgical care and burns hospital settings. Phages are used on their own or in combination with antibiotics and their use hasn’t been linked to any adverse effects.
With antibiotic-resistant infections becoming more common, bacteriophages offer the ability to treat such infections. But for bacteriophages to become commonplace in treating bacterial infections, there needs to be continued research into phage biology to better understand how they interact with bacteria. Finding effective treatments for bacterial infections – other than antibiotics – is the first step in fighting further instances of antibiotic resistance.
Manal Mohammed, Lecturer, Medical Microbiology, University of Westminster and Andrew Millard, Lecturer in Bacteriophage Bioinformatics, University of Leicester
This article is republished from The Conversation under a Creative Commons license. Read the original article.

